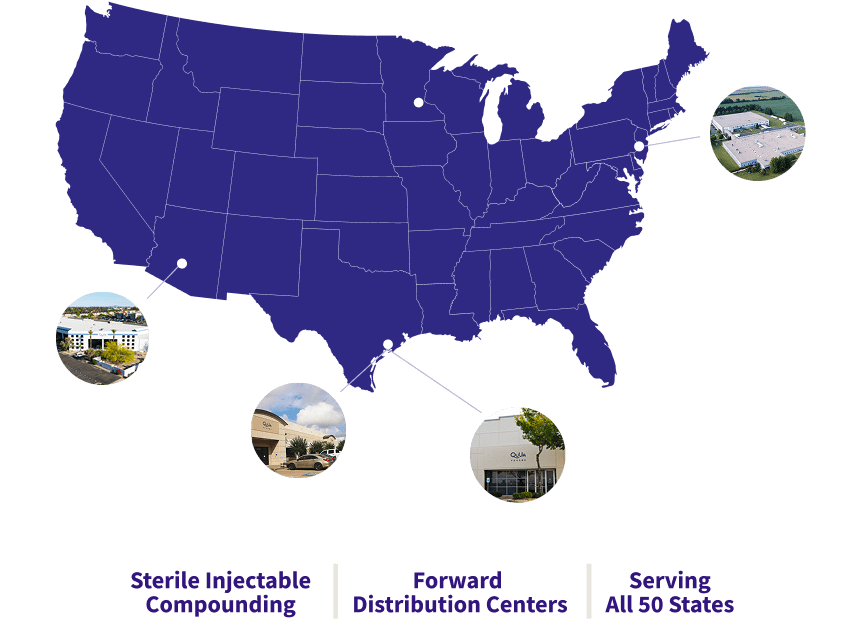
We have the capacity and scale to meet your demand now and in the future
Quva operates five state-of-the-art 503B outsourced sterile facilities with >300,000 sq ft of capacity in Texas, New Jersey, and Phoenix
Each Quva manufacturing facility has been completely purpose-built for 503B sterile compounding. Our industry-best manufacturing operations ensure that in Quva you have the highest quality extension of your pharmacy to support your sterile compounding needs.
See it. Believe it.
Quva’s facility tours have been designed to give you an inside look
at the processes, technology and people that allow Quva to set the
benchmark in 503B sterile compounding.
Bloomsbury Manufacturing Facility
165,000 sq ft
519 NJ-173,
Bloomsbury, NJ 08804
Sugar Land Manufacturing Facility
65,000 sq ft
1075 West Park One Drive, Suite 100,
Sugar Land, TX 77478
Quva Pharma, Inc.
Corporate Headquarters
3 Sugar Creek Center Blvd,
Suite 250
Sugar Land, TX 77478
Phone: (888) 339-0874
Phoenix Distribution Facility
44,000 sq ft
4717 East Hilton Avenue, Suite 400
Phoenix, AZ 85034
West Airport Production / Distribution Facility
75,000 Sq Ft
12620 W. Airport Blvd.
Sugar Land, TX 77478