Enhanced labeling design and placement
Quva’s ready-to-administer Oxytocin IV bags are updated with redesigned front and back labels, as well as front label placement that covers the diluent bag manufacturers linear barcode so that there is more clarity that Quva’s label barcodes are the ones scanned for the necessary compounded sterile product information like lot number, expiry, etc. This incorporates ISMP’s best practices on responsible oxytocin use, barcode verification, and medication safety.1
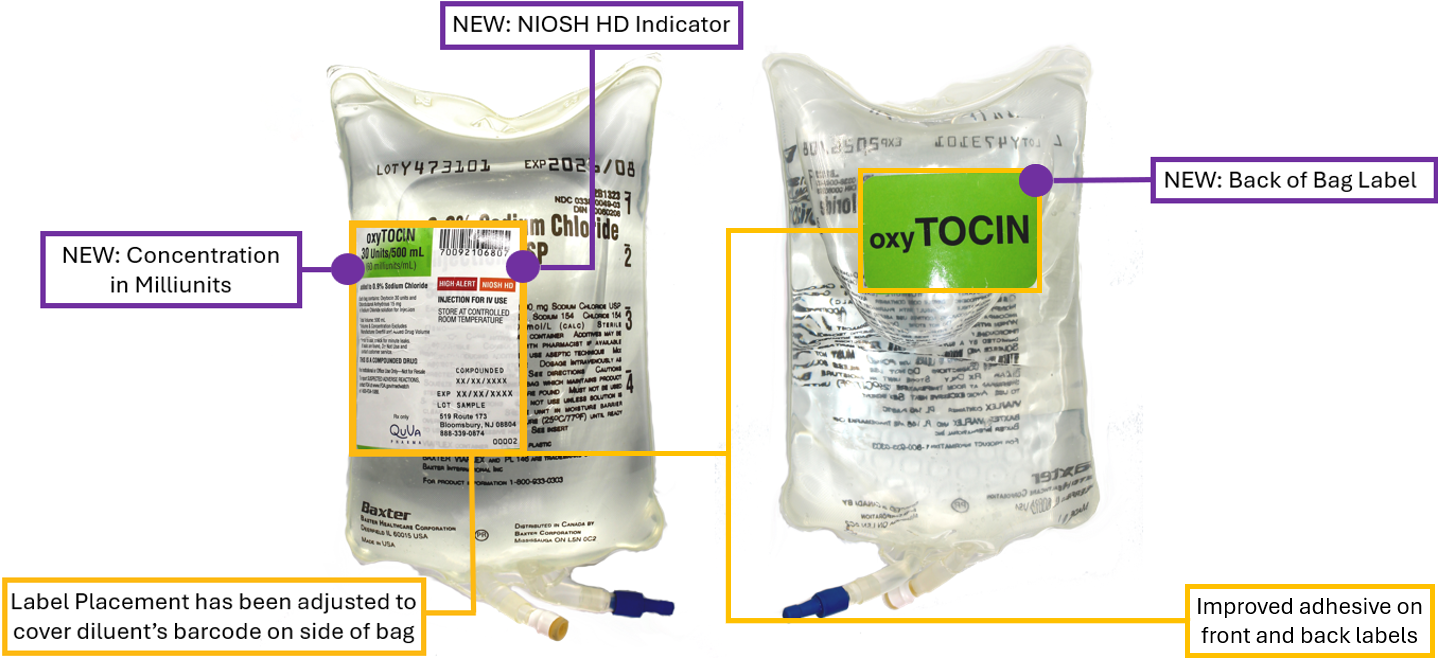

NEW: Front label redesign and enhancements
- Updated label placement and improved adhesive ensures secure coverage of the diluent bag’s barcode, reducing confusion and simplifying workflow by presenting a single barcode and expiration date of the compounded sterile product for scanning
- Concentration expressed in milliunits, supporting more precise titration in clinical settings and aligning with infusion pump dosing nomenclature
- NIOSH HD indicator added, expanding on the current High Alert designation and improving healthcare worker safety
NEW: Back auxiliary label
- Medication name displayed in TALLman lettering, increasing patient safety by aiding clear differentiation of oxytocin IV bags from plain hydrating solutions and magnesium infusions - An ISMP best practice
Focusing on Compliance, Safety, and Convenience
Purchasing ready-to-administer oxytocin is a key strategy employed by hospitals and health systems, with 56% of hospital pharmacies outsourcing oxytocin due to difficulties in compounding.2
Our products help:
- Reduce the risk of exposure to healthcare personnel, patients, and environment
- Satisfy both USP 797 compounding regulations and USP 800 regulations for handling hazardous drugs3
- Saves time and cost associated with compounding
Quva Pharma's Oxytocin Products
PRODUCT #
PRODUCT DESCRIPTION
PRODUCT SOURCE
BUD
(DAYS)
(DAYS)
CASE
QUANTITY
QUANTITY
70092106708
Oxytocin 20 Units added to 1000 ml 0.9 % Sodium Chloride Solution Bag
Sterile to Sterile
90
12
70092107025
Oxytocin 20 Units added to 1000 ml Lactated Ringer's Bag
Sterile to Sterile
45
12
70092106807
Oxytocin 30 Units added to 500 ml 0.9 % Sodium ChlorideSolution Bag
Sterile to Sterile
90
12
70092107124
Oxytocin 30 Units added to 500 ml Lactated Ringer's Bag
Sterile to Sterile
45
12
70092155207
Oxytocin 30 Units added to 500 ml 0.9 % Sodium Chloride Solution Bag (PF)
API to Sterile
60
12
Put patient safety first with our ready-to-administer Oxytocin products today!
DOWNLOAD PRODUCT INFORMATION SHEET
References
- 2024-2025 ISMP Targeted Medication Safety Best Practices for Hospitals. https://online.ecri.org/hubfs/ISMP/Resources/ISMP_TargetedMedicationSafetyBestPractices_Hospitals.pdf
- Pharmacy Purchasing and Products. Outsourced Compounding. https://www.pppmag.com/article/2911
- USP. Hazardous Drugs—Handling in Healthcare Settings. https://www.usp.org/compounding/general-chapter-hazardous-drugs-handling-healthcare
